Approval lapsed) RoActemra tocilizumab 200 mg/ 10mL concentrate for solution for infusion (Roche Germany) | Therapeutic Goods Administration (TGA)

Evaluation of the Tocilizumab therapy in human cancers: Latest evidence and clinical potential - Ghasemi - 2022 - Journal of Clinical Pharmacy and Therapeutics - Wiley Online Library
USTC Global on X: "The mechanism of using Tocilizumab to cure severe patients of COVID-19. @NIH @CDC @DrAiLynTan #coronavirus #COVID19 #CoronavirusOutbreak #coronaviruskorea #ItalyCoronavirus #italylockdown #IranCoVidTruth #Tocilizumab https://t.co ...
Approval lapsed) RoActemra tocilizumab 80mg/4mL concentrate for solution for infusion (Roche Germany) | Therapeutic Goods Administration (TGA)

Tocilizumab Actemra 400mg by cipla Roche at Rs 39000/vial | Tocilizumab Injection in Ahmedabad | ID: 25556328448







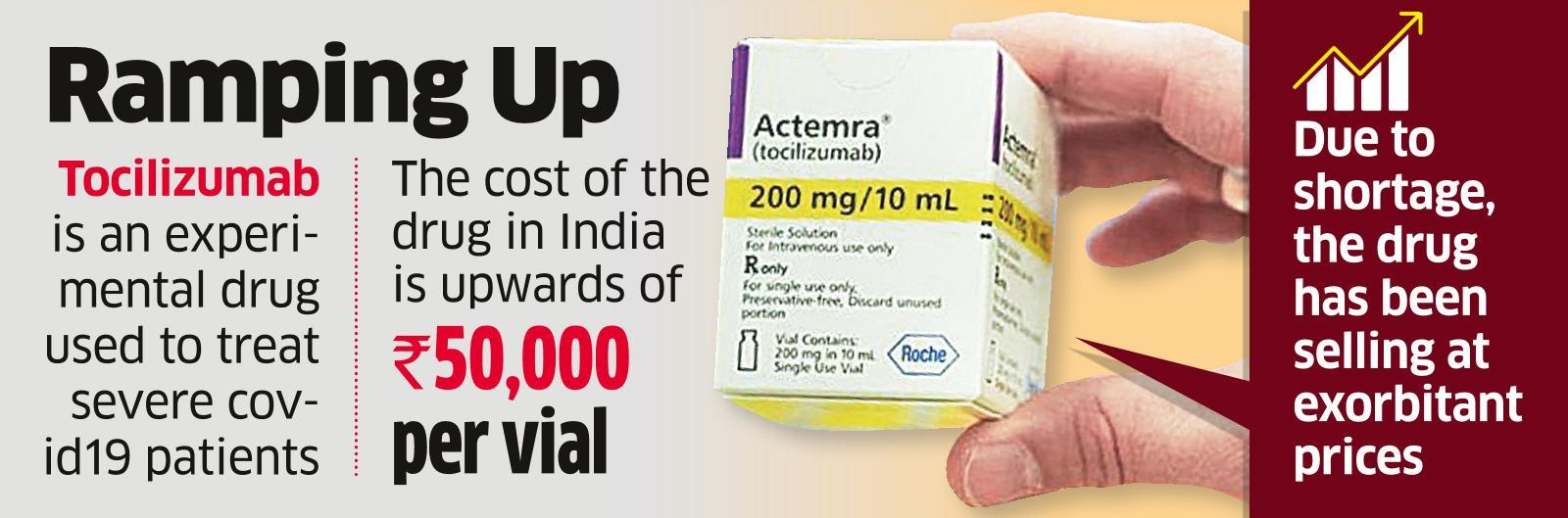

/cloudfront-us-east-2.images.arcpublishing.com/reuters/QFWUTH5BLZN5LGWVT27LGWPZBE.jpg)


:quality(90)/)